Our Work
The Advanced Retinal Imaging Alliance (ARIA) focuses its research on nine primary projects to develop systems and techniques used to better understand the physiology, structure and function of the human eye. These projects are spear-headed by teams of ARIA faculty, students, and expert research scientists and engineers with extensive experience, who develop entirely new technologies necessary to advance adaptive optics imaging development. ARIA's primary projects are:

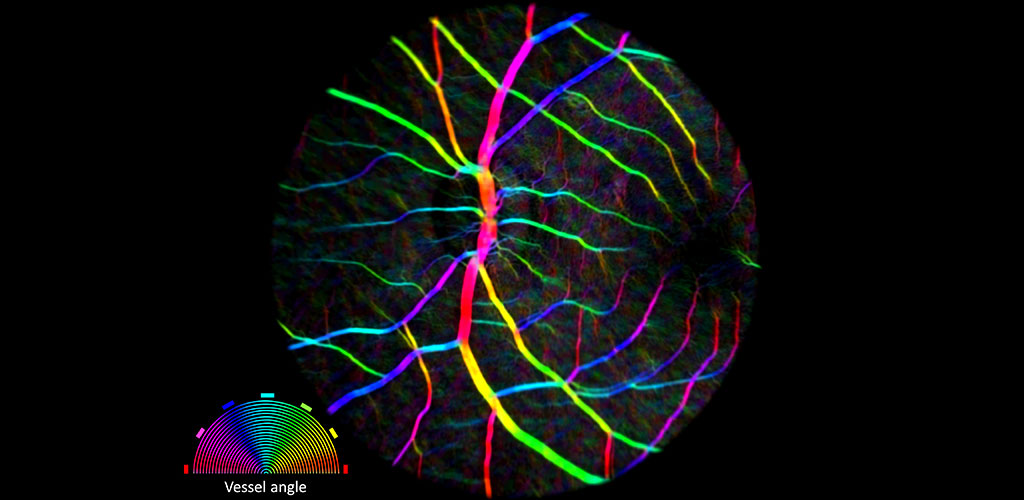
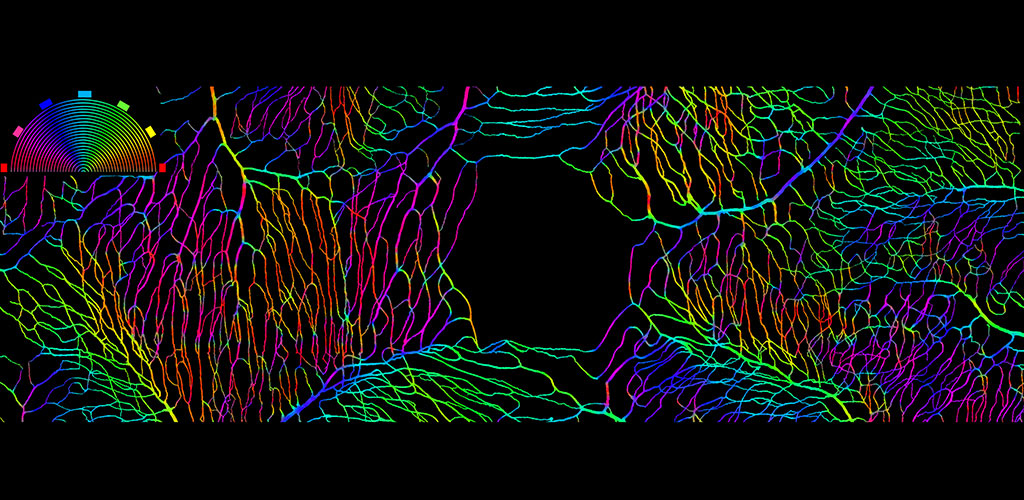
BLOOD FLOW: Neurovascular Coupling, Hemodynamic Imaging, Imaging Pericytes.
We are developing AOSLO technology to objectively report capillary blood flow by imaging the movement of single blood cells as they flow through the capillary network. This direct and non-invasive assessment of retinal blood flow provides structural and functional information on the vascular network of the retina. We are pursuing several projects in this area: 1) examining the role of capillary level neurovascular coupling in the central nervous system 2) characterizing flow and structure parameters that describe normal vascular perfusion and 3) exploring whether aberrant blood flow patterns can serve as a preclinical biomarker of early stages of retinal disease.
Read more »
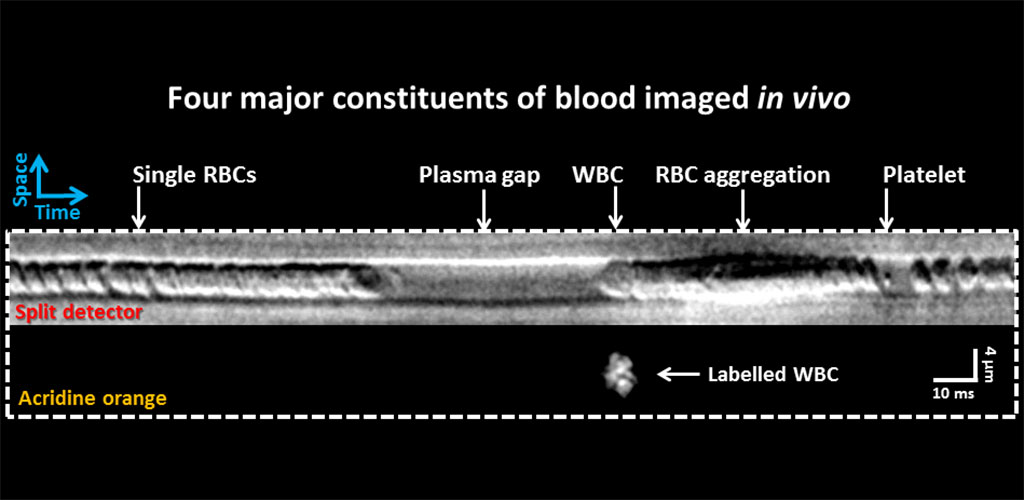
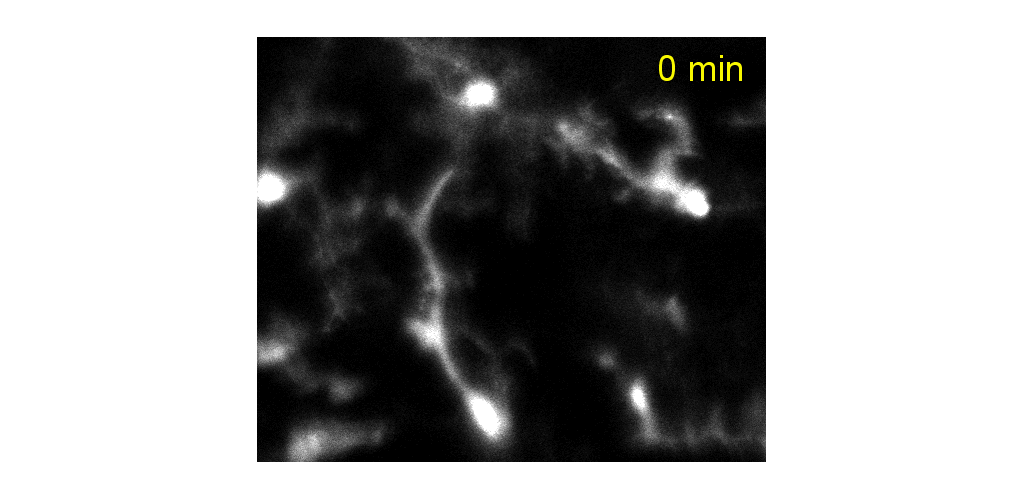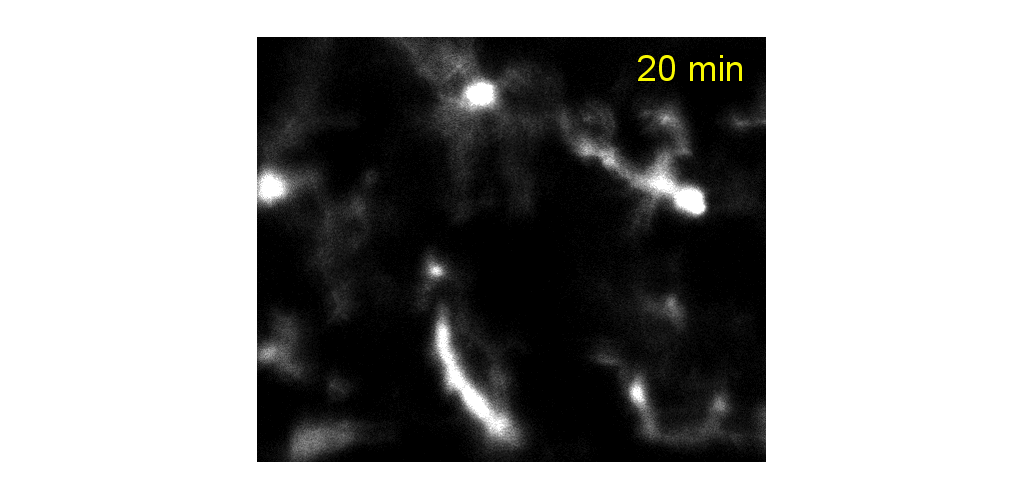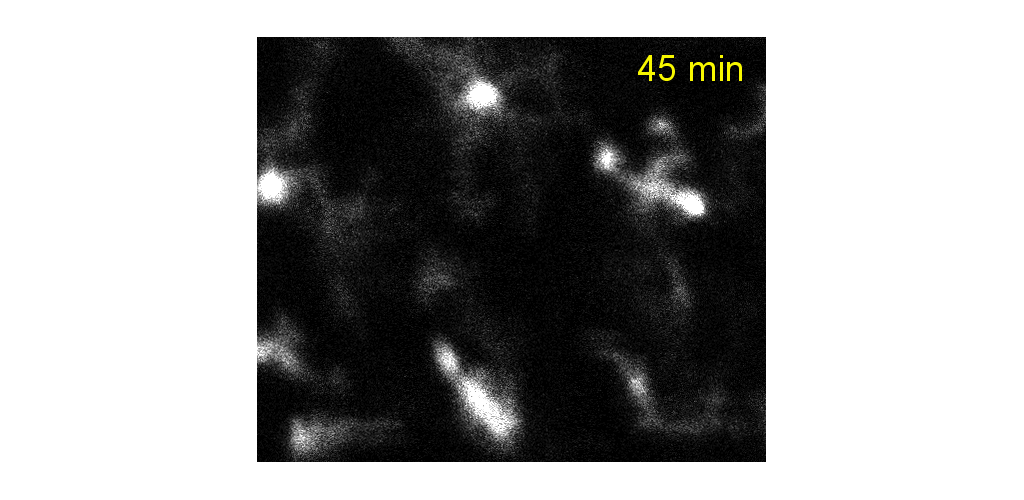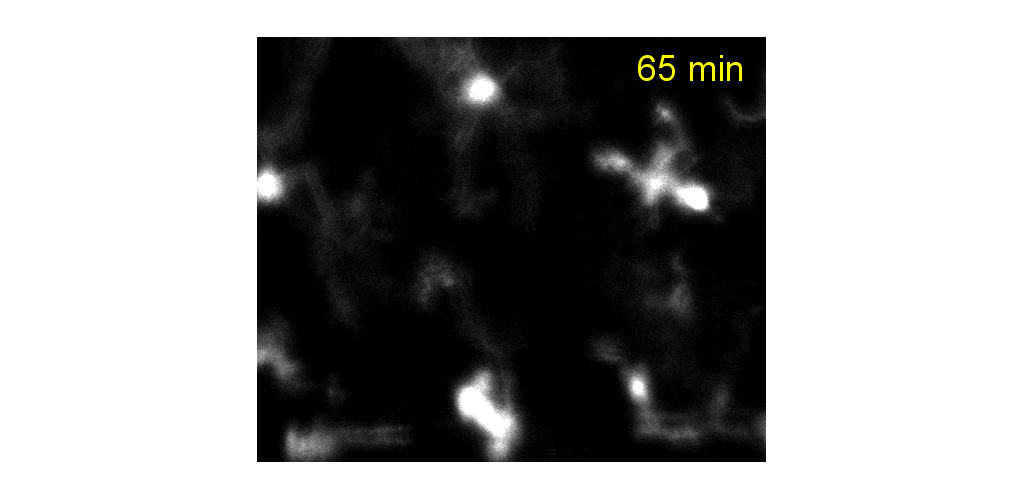
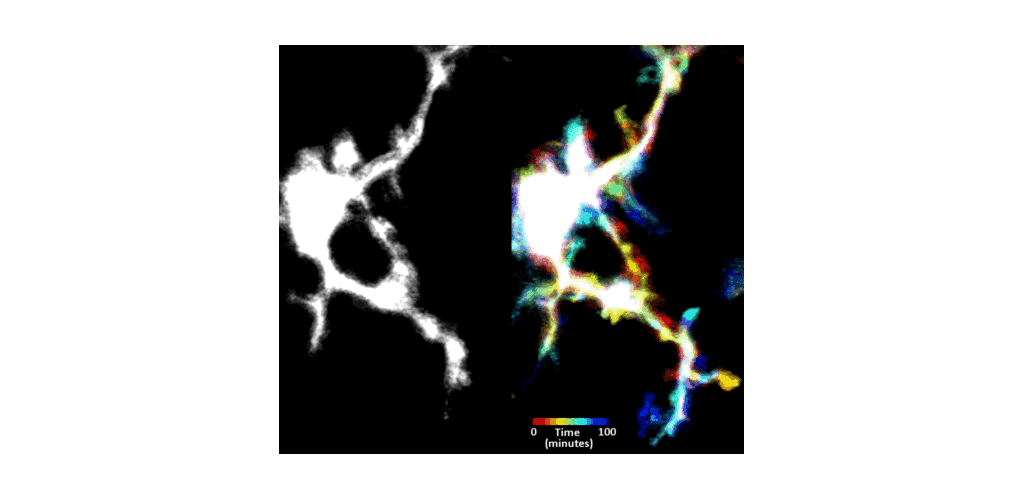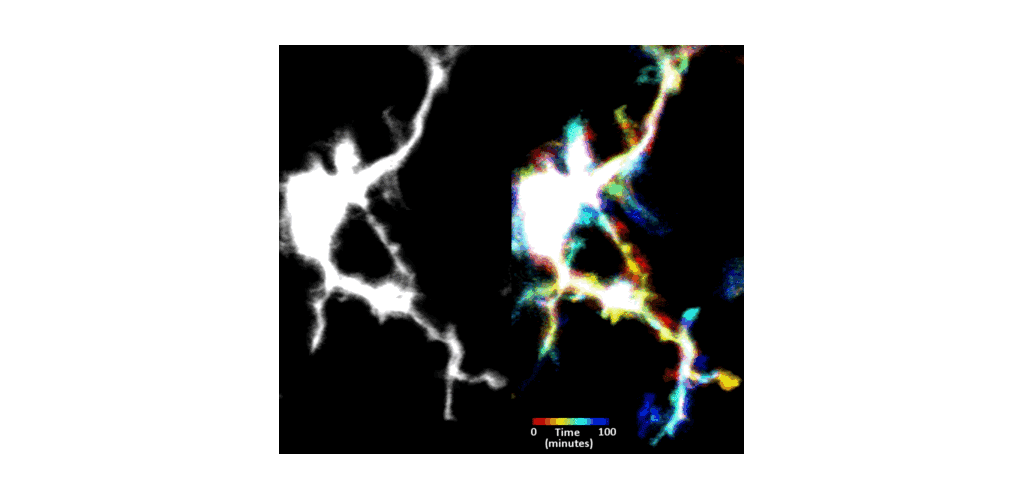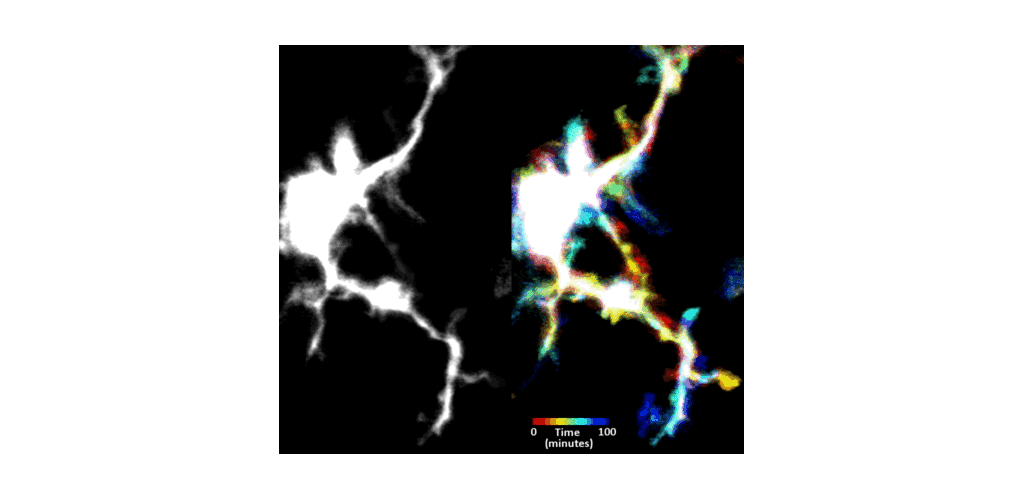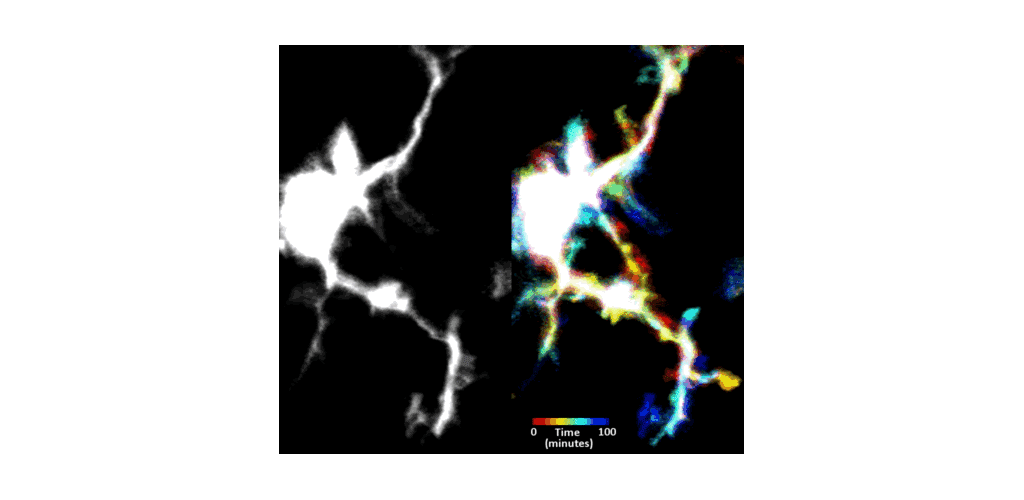
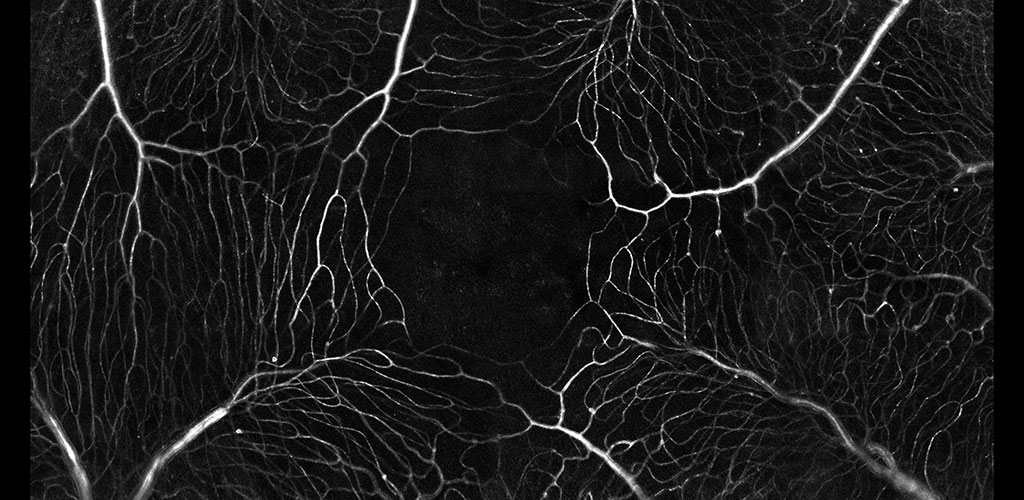
IMMUNE CELL IMAGING:
The retina, being a 'window' to the brain and the rest of the body, offers the opportunity to non-invasively study single cells of the immune system in their native micro-environment, and also inspect the global health of the body. Our lab's work on immune cell imaging examines the highly dynamic immune function from milliseconds-to-months in conditions of health and disease.
Read more »
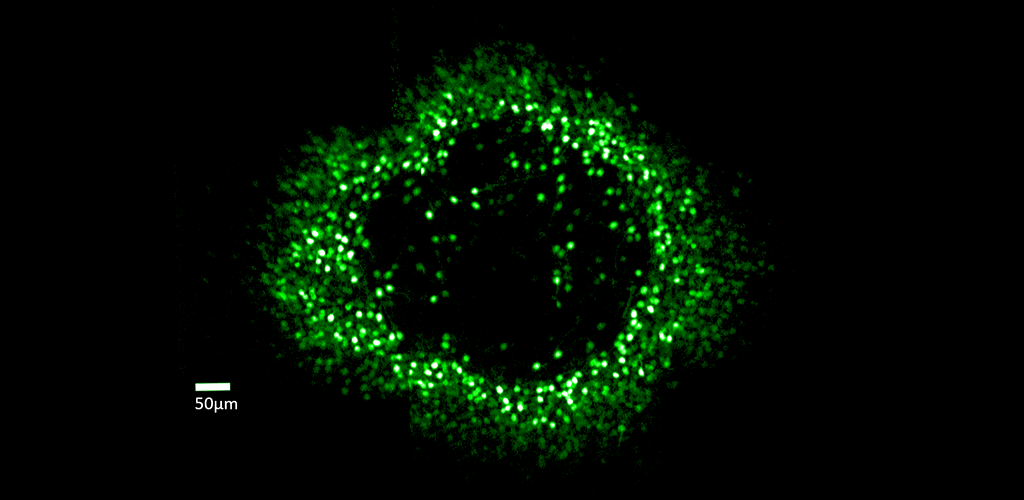
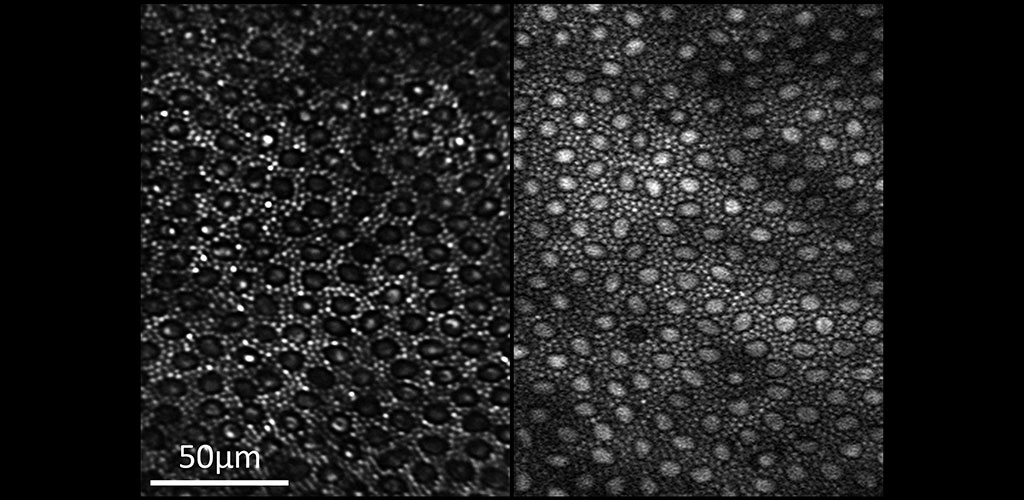
GANGLION CELL STRUCTURE & FUNCTION: Dr. Merigan's research examines the role of retinal ganglion cells in the visual perception of primate (human and macaque) and mouse. Although the retina contains more than 17 types of ganglion cell and each type forms a complete mosaic across the retina, little is known about what role each type plays in seeing. In collaboration with David Williams and Jennifer Hunter, Dr. Merigan is studying the role of different ganglion cell types using adaptive optics imaging of their calcium response.
Read more »
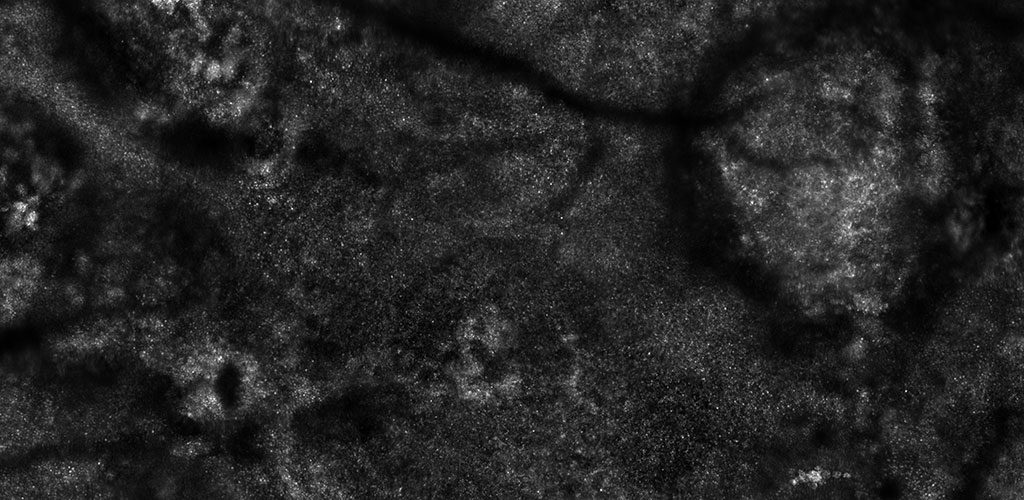
HUMAN RETINAL DISEASES: Adaptive optics imaging is a state of the art technology especially suited for non-invasive imaging of the human eye. Individual cells in multiple layers of the retina can be imaged through the dilated pupil. This technology can be used to discover the earliest indicators of retinal disease, follow disease progression over time and to provide quicker endpoints when studying the efficacy of treatments in clinical trials.
Read more »
Legacy Projects
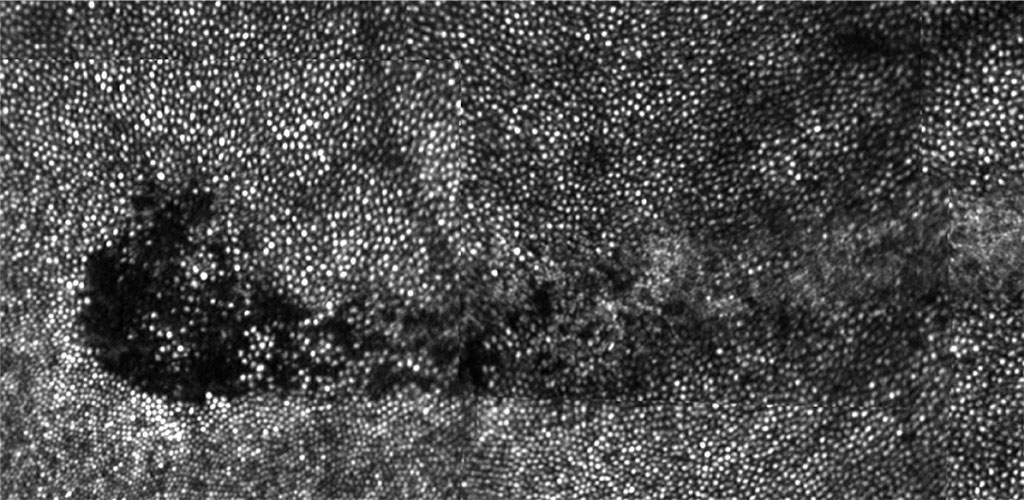
IN VIVO RETINAL PHOTOTOXICITY STUDY: We have discovered two new and unexpected effects on the primate retina caused by prolonged exposure to bright visible light (Morgan et al, 2008). The observed effects are autofluorescence (AF) photobleaching and, at higher light levels, retinal pigment epithelium (RPE) disruption. These effects raise interesting questions about how light interacts with the retina and if they are ultimately deleterious for the eye (Hunter et al, 2012).
Read more »