Blood Flow
Objective measures of blood flow in the retina
The neurons of the retina are one of the most metabolically active tissues in the human body. To serve this demand, a network of capillaries delivers nutrients and removes waste products from the highly metabolic neurons. Previously, the fine details of this capillary network have been obscured by insufficient spatial and temporal resolution. In this line of work, we are developing AOSLO technology to objectively report capillary blood flow by imaging the movement of single blood cells as they flow through the capillary network.
We are pursuing several projects in this area:
- examining the role of capillary level neurovascular coupling in the retina
- characterizing flow and structure parameters that describe normal vascular perfusion and
- exploring whether aberrant blood flow patterns can serve as a preclinical biomarker of early stages of retinal disease.
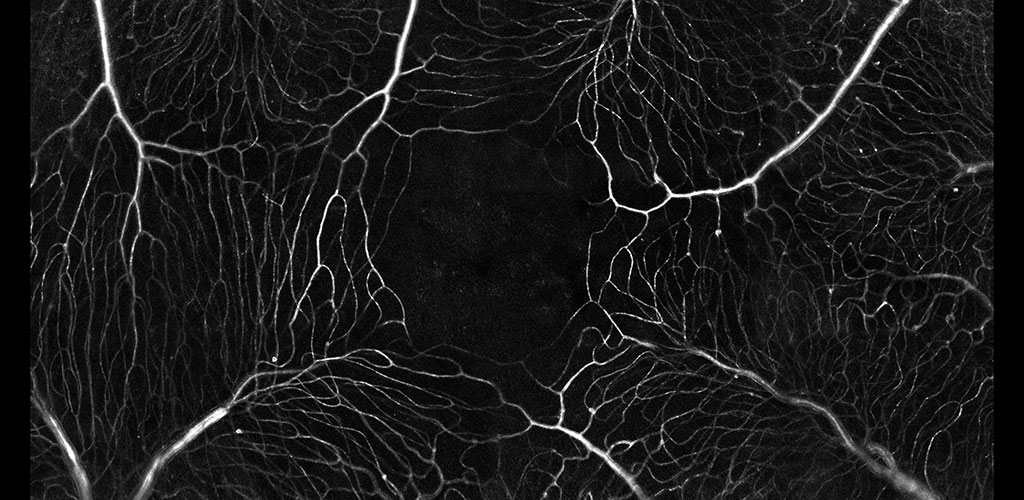
The microvascular network imaged without contrast agents. The movement of single blood cells creates a spatio-temporal flicker. Motion contrast imaging reveals active perfusion in the vessels surrounding the fovea.
Non invasive imaging of the microvascular network
We are developing a new methodology which precludes the need for extrinsic contrast agents. Using the intrinsic spectral and scatter properties of individual blood cells, we capture the movement of these cells in video rate adaptive optics. The movement of these cells provides a map of blood motion. Blood vessels contain strong motion (bright) whereas static features such as neurons and glia produce little motion (dark). The resultant perfusion maps provide functional information on capillary perfusion.

Objective measurements of blood flow
In this project, we are developing automated ways to directly measure and report the speed of individual blood cells as they move through the retinal circulation.
In the image above, blood cells flow in a single capillary path is imaged without contrast agents. Movement of single blood cells can be tracked over time. Change in cell displacement, dx, divided by change in time, dt reveals the velocity of cells. Blood velocities ~0.3-3 mm/second were measured by this method.
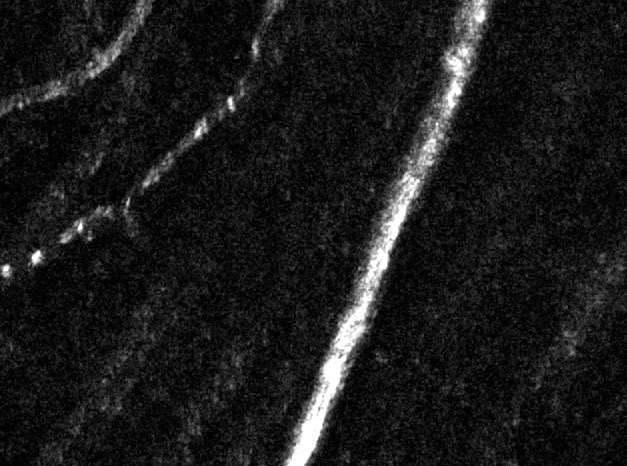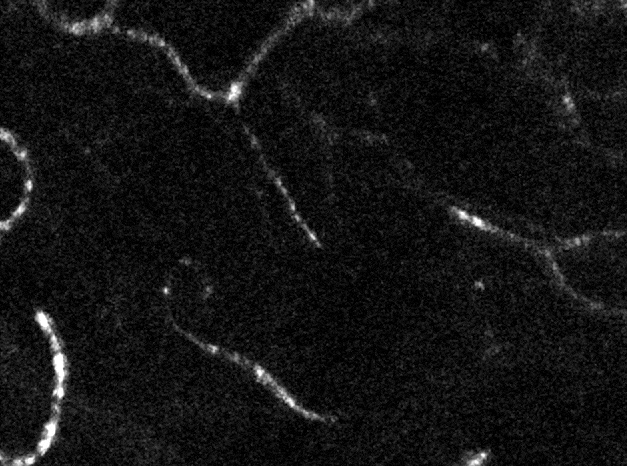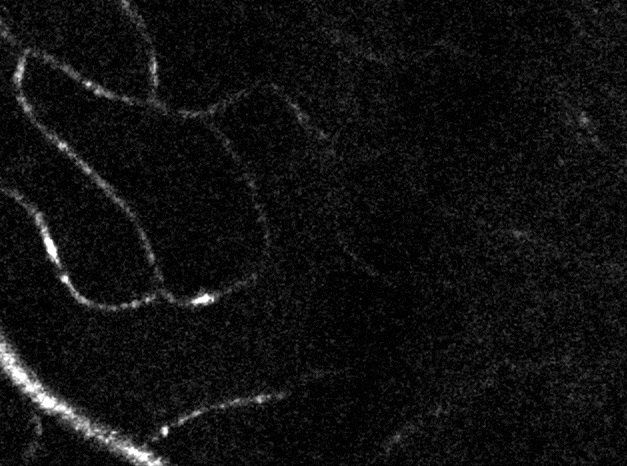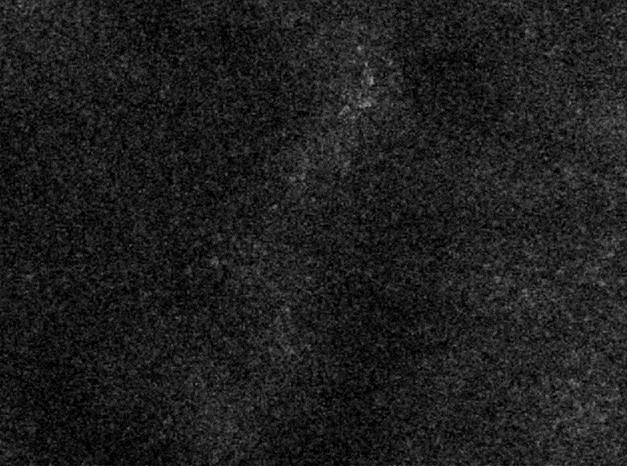
Confocal imaging in vivo
The AOSLO is a confocal instrument. By dynamically changing the depth of focus, we can optically section the retina. In the above image we can distinguish the location of three levels of capillaries in the retinal circulation. Image field is 5 degrees of visual angle, ~150 microns across.
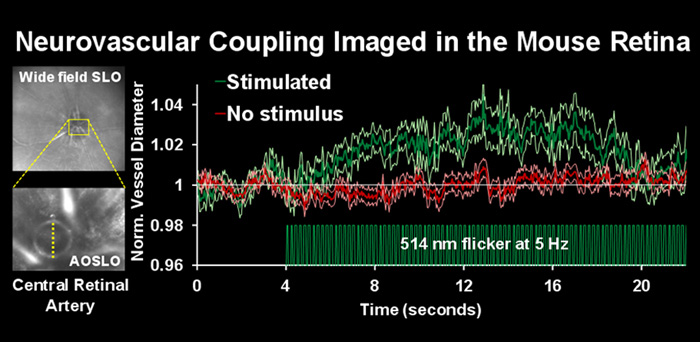
Imaging blood flow dynamics in response to neural stimulation
In the central nervous system, increased levels of neural activity are associated with an increase in local blood flow. The circuit that mediates increase in blood flow is poorly understood because the neurons, glia and vascular cells reside deep in the opaque recesses of the brain. In this study, we attempt to image the neurovascular unit non-invasively in the retina, through the optical window of the eye.
Confocal AOSLO reveals the bore of the central retinal artery en face using NIR, 796nm light. The diameter of the central retinal artery was measured with and without visual stimulation. After presentation of flickering light, there was an increase of central retinal artery diameter of over 3% (green trace). There was no dilation in the absence of visual stimulation (red trace).
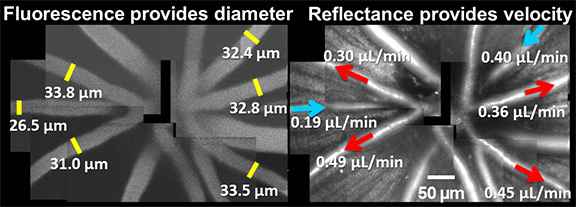
Quantifying total blood flow in the retina
With the goal of studying vascular abnormalities in the eye in diseases like diabetes, our lab has developed a novel way of non-invasively tracking the total volumetric flow rate of blood in the living retina using adaptive optics scanning light ophthalmoscopy (AOSLO) (Joseph et al. 2019). This technique automatically determines blood cell velocity with a custom algorithm (Schallek 2017 patent). Blood cell velocity and vessel diameter together give precise measures of volumetric blood flow. The figure below shows an example of such an in vivo determination of total blood flow in the mouse retina.
Quantifying pulsatile and laminar flow in vessels of all size: capillaries-to -venules/arterioles
Single blood cell measurements of blood velocity show fine modulations in response to cardiac pressure wave and laminar profile. We are developing algorithms to automatically measure and report hemodynamic biomarkers of flow rate (µL), pulsatility index and laminar flow variation. We are evaluating these biomarkers in label-free blood cell imaging to report small changes in retinal blood flow in retinal vascular diseases like diabetic retinopathy (DR). These early measurements may someday predict if a patient has a high risk of developing advanced retinal vascular disease.
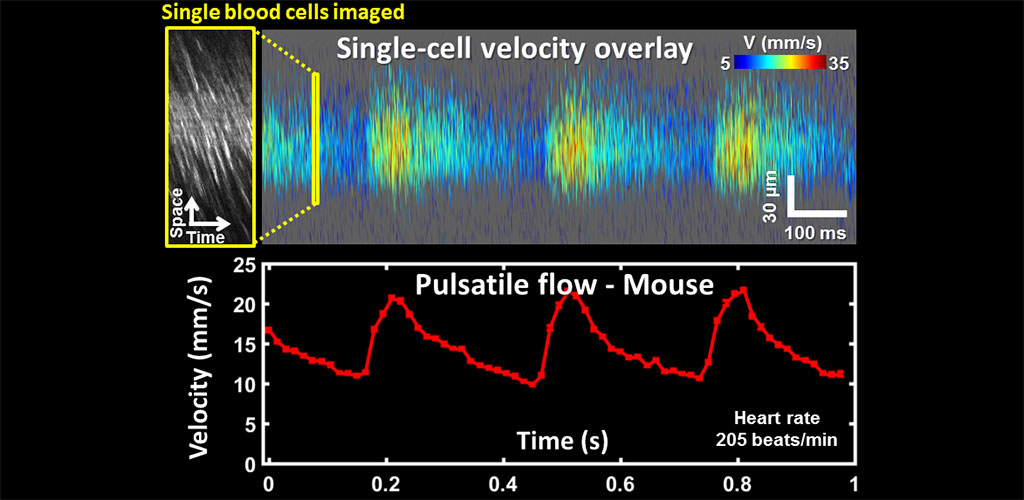
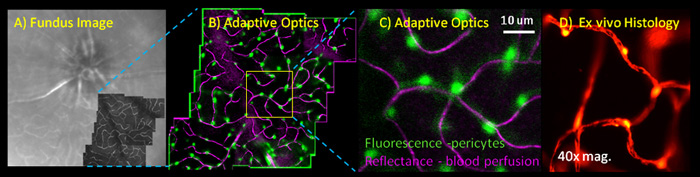
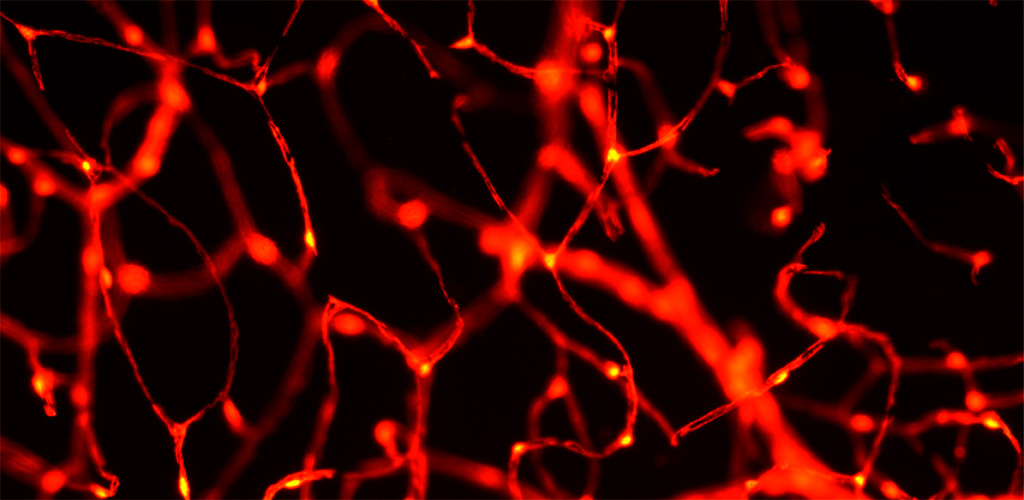
Image shows extrinsically labeled pericytes for the Neural-glial antigen 2. Images are from A) confocal Scanning laser ophthalmoscope. B) 2-channel adaptive optics showing capillary perfusion (magenta) and NG2+ pericytes (green). C) High resolution image of a single AOSLO field. D) Validation of pericyte labeling in post-mortem histology.
Characterizing pericyte density and regulation of blood flow in the mouse retina in vivo
Schallek et al. IOVS 2013 (pdf)
Pericytes are vascular associated cells that have been implicated as mediators of blood brain barrier formation, capillary structural scaffolds, and more recently, have implicated in regulating blood flow at the level of capillaries. However, many of these functions have been inferred from post mortem histology as single pericytes are very small (<7 microns in diameter) and offer poor optical contrast in the native retina. In this line of work, we are investigating the structural and functional roles of pericytes in the living animal.
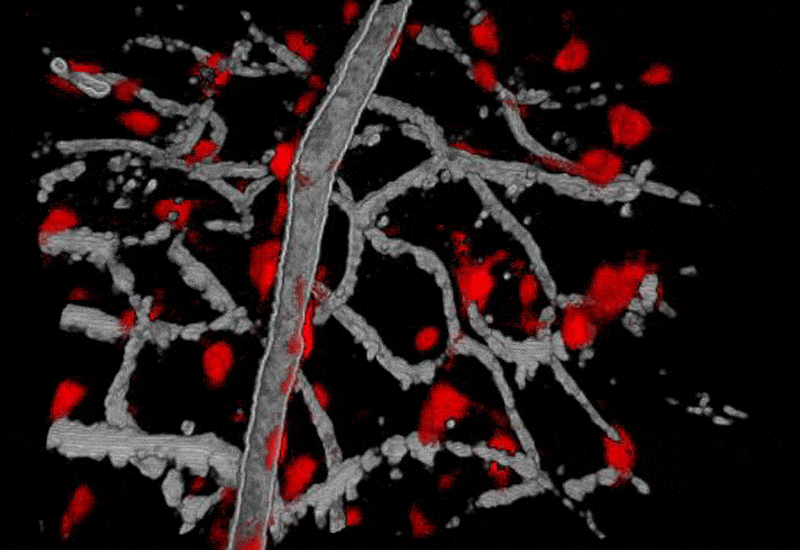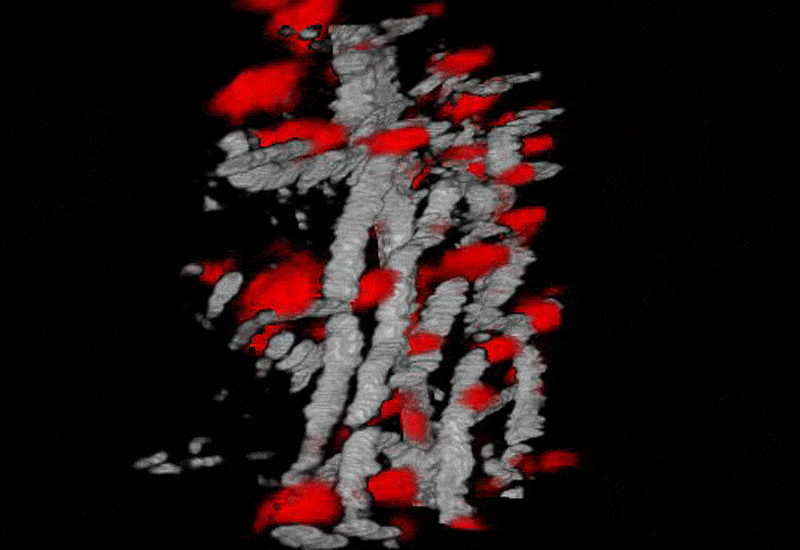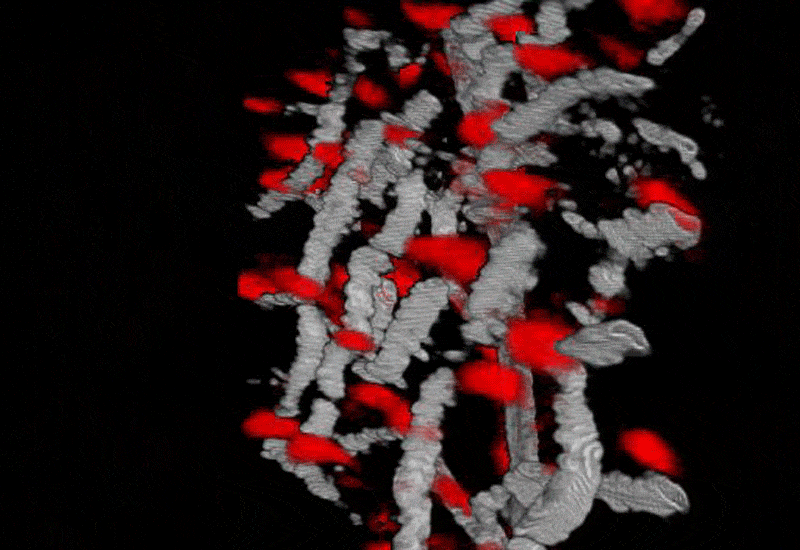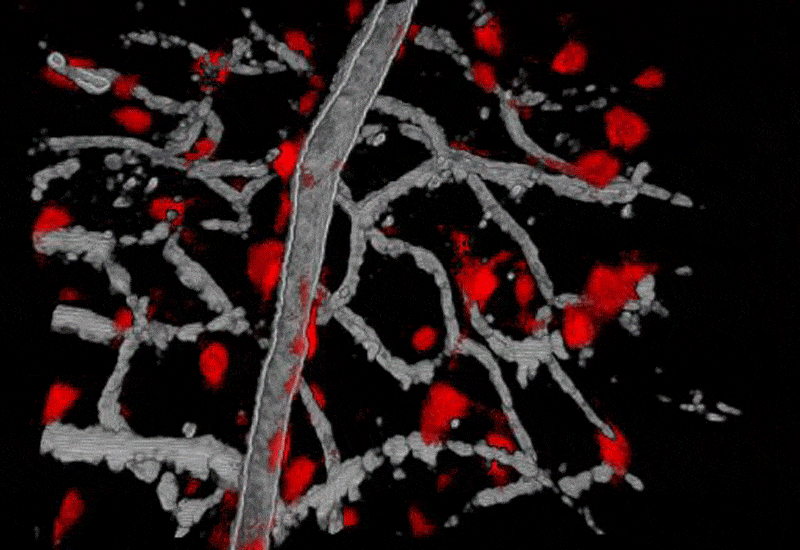
To overcome previous limitations of resolution and optical contrast we image NG2 transgenic mice which have fluorescent pericytes in the brain and retina with AOSLO technology. To image these fluorescent cells, we have equipped the AOSLO with a second channel that allows fluorescence imaging simultaneously with the infrared reflectance imaging described above. In this line of work, we are characterizing the role of pericytes in providing more blood to active neural regions (neurovascular coupling) and characterizing the role of pericyte loss in animal models of human diabetes.
Funding Sources
- NIH NEI R01 EY028293-01 Title: Non-invasive, living histology of capillary structure and single cell blood flow in a mouse model of diabetic retinopathy. Jesse Schallek, PI
- Dana Foundation- David Mahoney Neuroimaging Grant: Jesse Schallek, PI
- Research to Prevent Blindness Career Development Award Jesse Schallek, PI
Publications
- Shang F, Schallek J (2024). Characterization of the Retinal Circulation of the Mouse. Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):3. doi: 10.1167/iovs.65.14.3. PMID: 39620830.
- Feng G, Joseph A, Dholakia K, et al. (2023) High-resolution structural and functional retinal imaging in the awake behaving mouse. Commun Biol 6, 572. https://doi.org/10.1038/s42003-023-04896-x
- Feng G, Kunala K, Yang Q, Schallek J (2023). Quantitative phase and volumetric retinal imaging using angle-resolved AOSLO imaging. Proc. SPIE 12360, Ophthalmic Technologies XXXIII, 1236009; https://doi.org/10.1117/12.2647024
- Dholakia KY, Guevara-Torres A, Feng G, Power D, Schallek J (2022). In Vivo Capillary Structure and Blood Cell Flux in the Normal and Diabetic Mouse Eye. Investigative Ophthalmology & Visual Science 63, 18. doi:10.1167/iovs.63.2.18 PDF
- Joseph, A., Guevara-Torres, A., and Schallek, J. (2019). Imaging single-cell blood flow in the smallest to largest vessels in the living retina. ELife 8, e45077. PDF
- Guevara-Torres A, Joseph A, & Schallek JB (2016). Label free measurement of retinal blood cell flux, velocity, hematocrit and capillary width in the living mouse eye. Biomed. Opt. Express, BOE 7, 4228–4249. PDF
- Guevara-Torres A, Williams DR, and Schallek JB (2015). Imaging translucent cell bodies in the living mouse retina without contrast agents. Biomed. Opt. Express 6, 2106-2119. PDF
- Schallek J., Geng, Y., Nguyen, H., and Williams, D.R. (2013). Morphology and topography of retinal pericytes in the living mouse retina using in vivo adaptive optics imaging and ex vivo characterization. Invest Ophthal Vis Sci. PDF