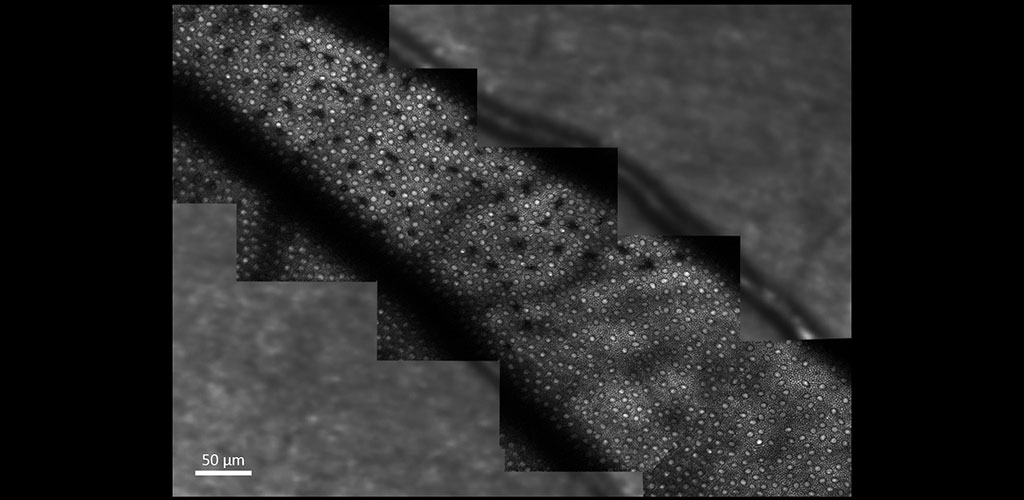
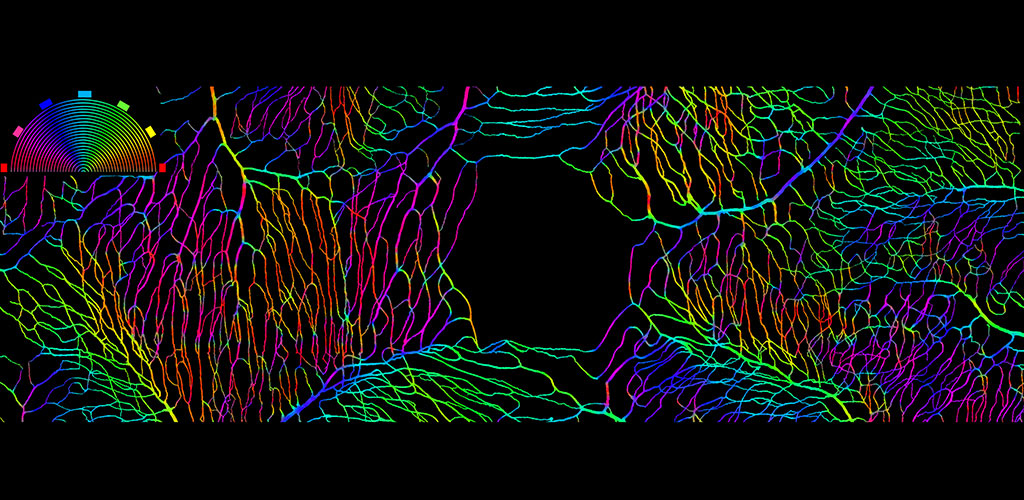
Overview
ARIA uses adaptive optics imaging of retinal elements to elucidate the structure and function of the human eye. We are a multidisciplinary lab comprised of researchers from the Flaum Eye Institute, the Center for Visual Science and The Institute of Optics at the University of Rochester. This bridge between the engineering and medical worlds, due to the geographic proximity of the world-renowned Optics Institute and the growing Flaum Eye Institute, provides a unique environment for research. Our group is passionate about understanding the physiology underlying healthy and diseased visual systems.
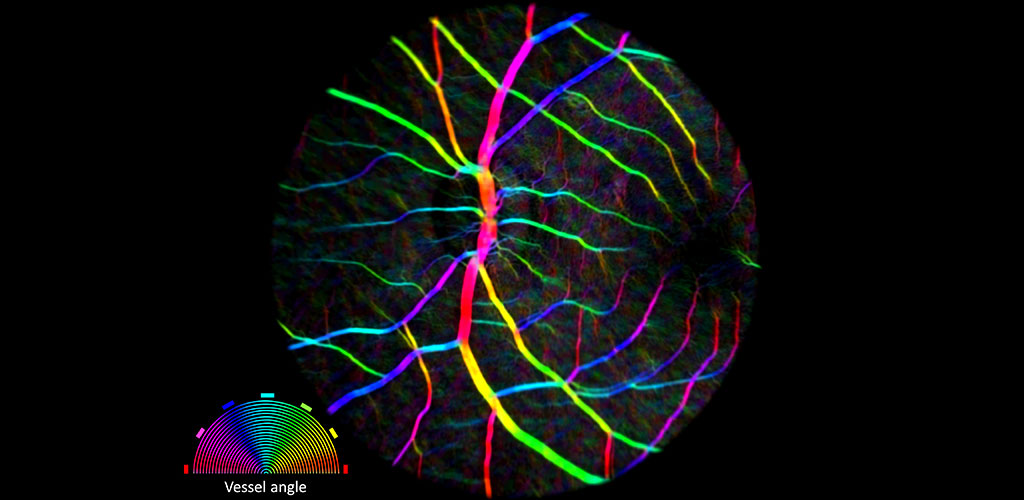
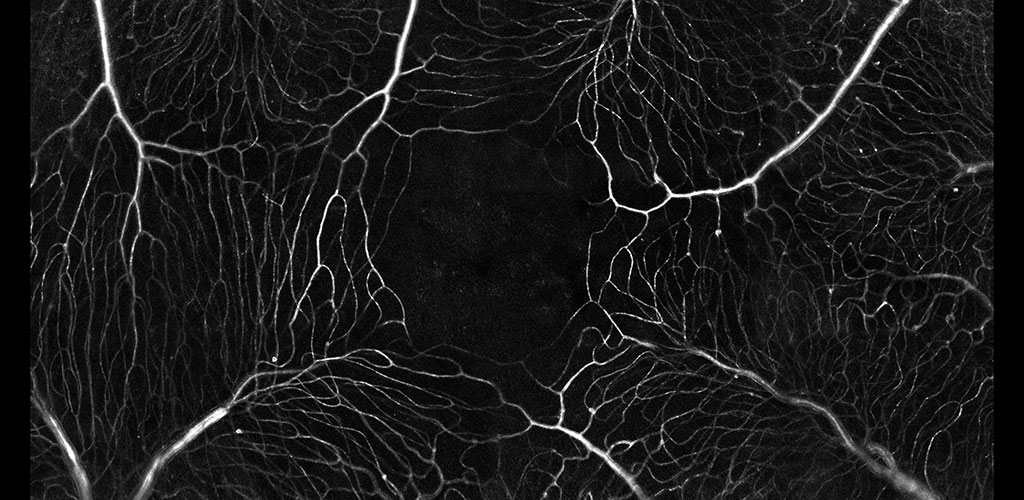
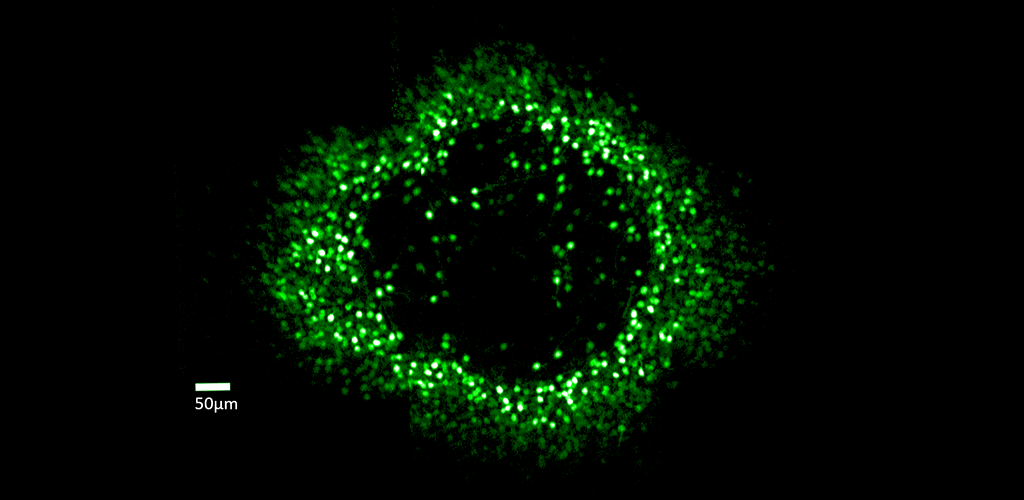
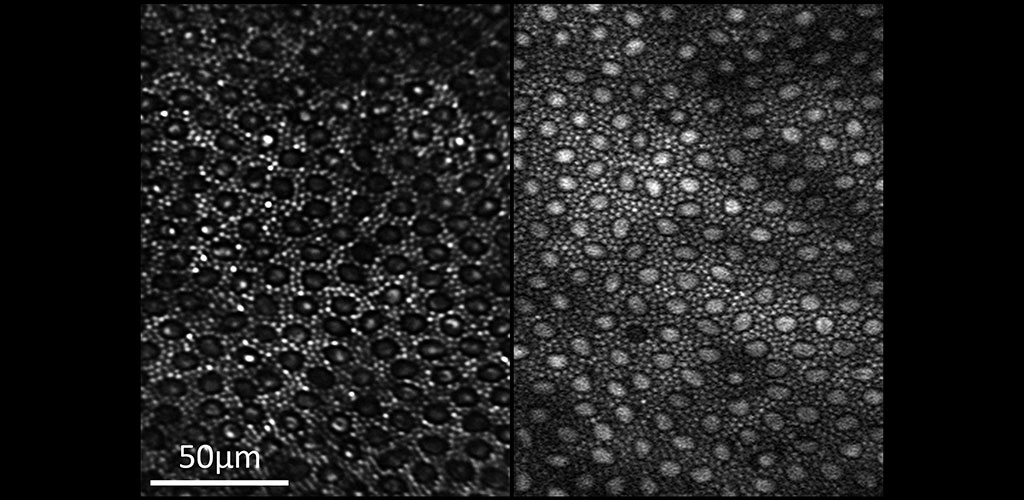
From our research, we have been able to non-invasively see structures such as capillary blood flow and photoreceptor cells in extraordinary detail. This cellular and sub-cellular information is useful in understanding disease progression and prevention, and for informing therapeutic strategies. As methods and systems are advanced, it may be possible to more accurately diagnose retinal diseases at an earlier time point and to more effectively target treatment, as well as to better understand the cellular etymology and pathways of many disorders.