Ganglion Cell Structure and Function
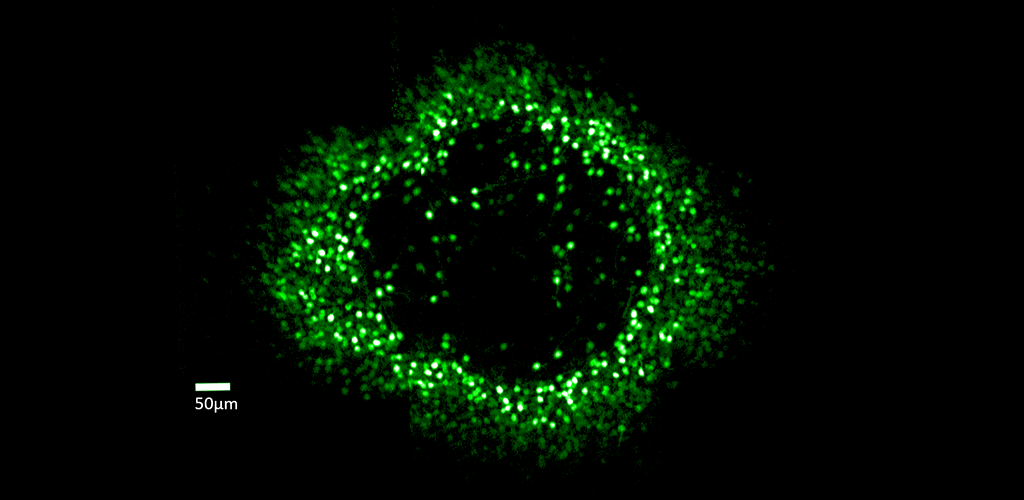
Fluorescent ring of ganglion cells serving the foveal cones. These cells are expressing the calcium indicator GCaMP, which allows cell responses to be monitored optically.
In vivo measurements of the change in fluorescence of GCaMP6s-expressing foveal ganglion cells in response to visual stimuli
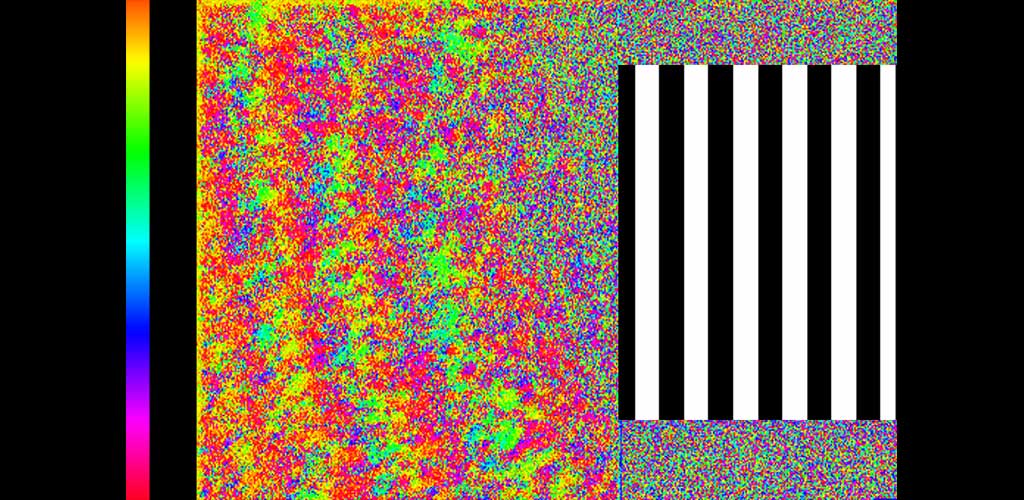
Phase map of ganglion cell responses to a 0.2Hz drifting grating visual stimulus presented to the foveal cones.
This research examines the role of retinal ganglion cells in the visual perception of primate (human and macaque) and mouse. Although the retina contains more than 17 types of ganglion cells and each type forms a complete mosaic across the retina, little is known about what role each type plays in seeing. ARIA is studying the role of different ganglion cell types using adaptive optics imaging of their calcium response.
- Anatomical classification of fluorescently-labeled ganglion cells in the living eye.
- Development of super resolution imaging capabilities for imaging ganglion cell dendritic fields in the living eye.
- Computational models of cone inputs to foveal ganglion cells.
- Imaging the physiological activity of retinal ganglion cells with G-CaMP, a calcium indicator.
- Restoration of vision to blind retina by inserting channel rhodopsin into retinal ganglion cells. Determines how perception can be mediated by light-gated channels used to restore vision to blind subjects.
- Identification and classification of retinal ganglion cells using response characteristics to chromatic and spatio-temporal stimuli.
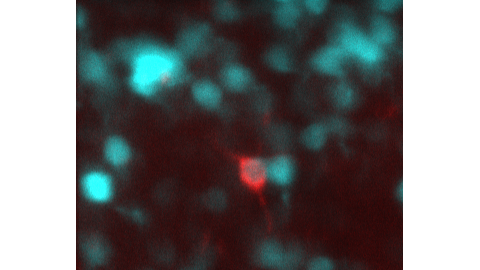
Blue: foveal ganglion cells expressing GCaMP6s. Red: a ganglion cell retrogradely-labeled from the superior colliculus. These rarer ganglion cells have never been studied in the fovea
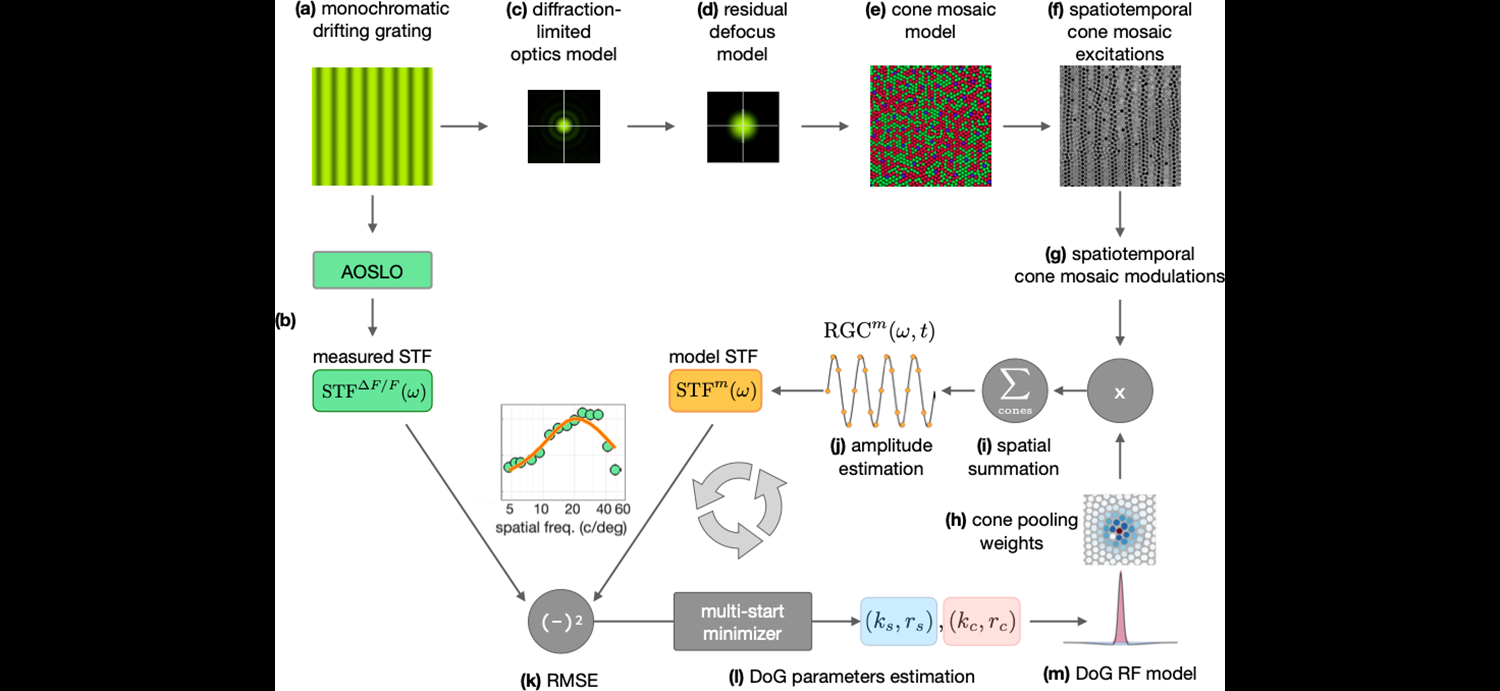
Computational model of pooling of cone inputs to foveal midget ganglion cells using ISETBio
Funding Sources
- R01-EY021166, National Eye Institute, “Physiological and perceptual examination of vision restoration”, PI: Bill Merigan
- R01-EY031467, National Eye Institute, “High Resolution Mapping of Foveal Receptive Fields in the Living Primate Eye”, PI: David Williams and Bill Merigan
- FA9550-22-1-0167, Air Force Office of Scientific Research, “Single Retinal Ganglion Cells and Sensation”, PI: David Williams
- FA9550-22-1-0044, Air Force Office of Scientific Research, “Super Resolution Adaptive Optics Ophthalmoscope for Revealing the Retinal Code”, PI: David Williams
- F32-EY032318, National Eye Institute, “Foveal Ganglion Cell Function in the Living Eye”, PI: Sara Patterson
Publications
- Godat T, Cottaris NP, Patterson S, Kohout K, Parkins K, Yang Q, et al (2022). In vivo chromatic and spatial tuning of foveolar retinal ganglion cells in Macaca fascicularis. PLoS ONE 17(11): e0278261. https://doi.org/10.1371/journal.pone.0278261
- McGregor JE, Kunala K, Xu Z, Murphy P, Godat T, Strazzeri JM, Bateman B, Fischer W, Puthussery T, Williams DR, Merigan WH (2021). Optogenetic therapy restores retinal activity in primate for at least a year following photoreceptor ablation. Molecular Therapy, 1525-0016, https://doi.org/10.1016/j.ymthe.2021.09.014. PDF
- McGregor JE, Godat T, Dhakal KR, Parkins K, Strazzeri JM, Bateman BA, Fischer WS, Williams DR, Merigan WH (2020). Optogenetic restoration of retinal ganglion cell activity in the living primate. Nature Communications 11(1), 1703. PDF
- McGregor, J.E. (2019). Restoring vision at the fovea. Current Opinion in Behavioral Sciences 30, 210-216. PDF
- McGregor JE, Yin L, Yang Q, Godat T, Huynh KT, Zhang J, Williams DR, Merigan WH (2018). Functional architecture of the foveola revealed in the living primate. PLoS One 13(11):e0207102. PDF
- Yin L, Masella B, Dalkara D, Zhang J, Flannery JG, Schaffer DV, Williams DR, Merigan WH (2014). Imaging light responses of foveal ganglion cells in the living macaque eye. J Neurosci. ;34(19):6596-605. doi: 10.1523/JNEUROSCI.4438-13.2014. PDF
- Yin L, Geng Y, Osakada F, Sharma R, Cetin AH, Callaway EM, Williams DR, Merigan WH (2013). Imaging light responses of retinal ganglion cells in the living mouse eye. J Neurophysiol. 2013 Feb 13. PDF
- Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV (2013). In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 5(189):189ra76. PDF
- Geng, Y., Dubra, A., Yin, L., Merigan, W.H., Sharma, R., Libby, R.T., and Williams, D.R. (2012). Adaptive optics retinal imaging in the living mouse eye. Biomed Opt Express 3(4), 715-734. PDF
- Yin, L., Greenberg, K., Hunter, J.J., Dalkara, D., Kolstad, K.D., Masella, B.D., Wolfe, R., Visel, M., Stone, D., Libby, R.T., DiLoreto, Jr., D., Schaffer, D.V., Flannery, J., Williams, D.R., Merigan, W.H. (2011). Intravitreal injection of AAV2 transduces macaque inner retina. IOVS. 52(5):2775-2783. PDF
- Scoles, D., Gray, D.C., Hunter, J.J., Wolfe, R., Gee, B.P., Geng, Y., Masella, B.D., Libby, R.T., Russell, S., Williams, D.R., Merigan, W.H. (2009). In vivo imaging of retinal nerve fiber layer vasculature: imaging – histology comparison. BMC Ophthalmology, 9:9 doi:10.1186/1471-2415-9-9. PDF
- Gray, D.C., Wolfe, R., Gee, B.P., Scoles, D., Geng, Y., Masella, B.D., Dubra, A., Luque, S., Williams, D.R., Merigan, W.H. (2008). In vivo imaging of the fine structure of rhodamine labeled macaque retinal ganglion cells. IOVS, 49(1), 467-73. PDF
- Merigan, W.H, Freeman, A., Meyers, S. (1997). Parallel processing streams in human visual cortex. Neuroreport, 8, 3985-3991.
- Merigan, W.H. and Maunsell, J.H.R. (1993). How parallel are the primate visual pathways. Annual Review of Neuroscience, 16, 369-402.