Optogenetic Vision Restoration
Optogenetic reversal of vision loss in the macaque
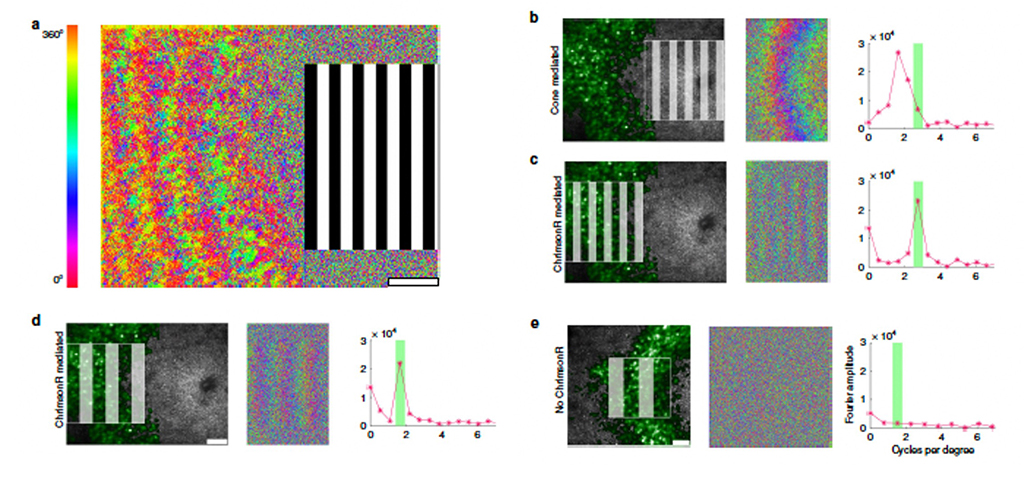
Figure 1 (From McGregor et al, 2020)
As figure 1 shows, our laboratory has demonstrated substantial vision recovery in otherwise blind retinal ganglion cells (RGCs) with use of the 3rd generation optogenetic, ChrimsonR (Klapoetke et. al., 2014). a. shows the center foveal region of stimulation by a drifting grating and the RGC response (color shows phase angle) to the left. b. shows the same result in 3 panels, for a retina with no cone lesion, left is the grating and green labelled RGCs, mid is the phase angle response and right is the spatial frequency response (red triangles) and grating spatial frequency in green. c and d show the response when the central cones are lesioned for a higher and lower spatial frequency grating, and e a control condition in which he optogenetic is no present.
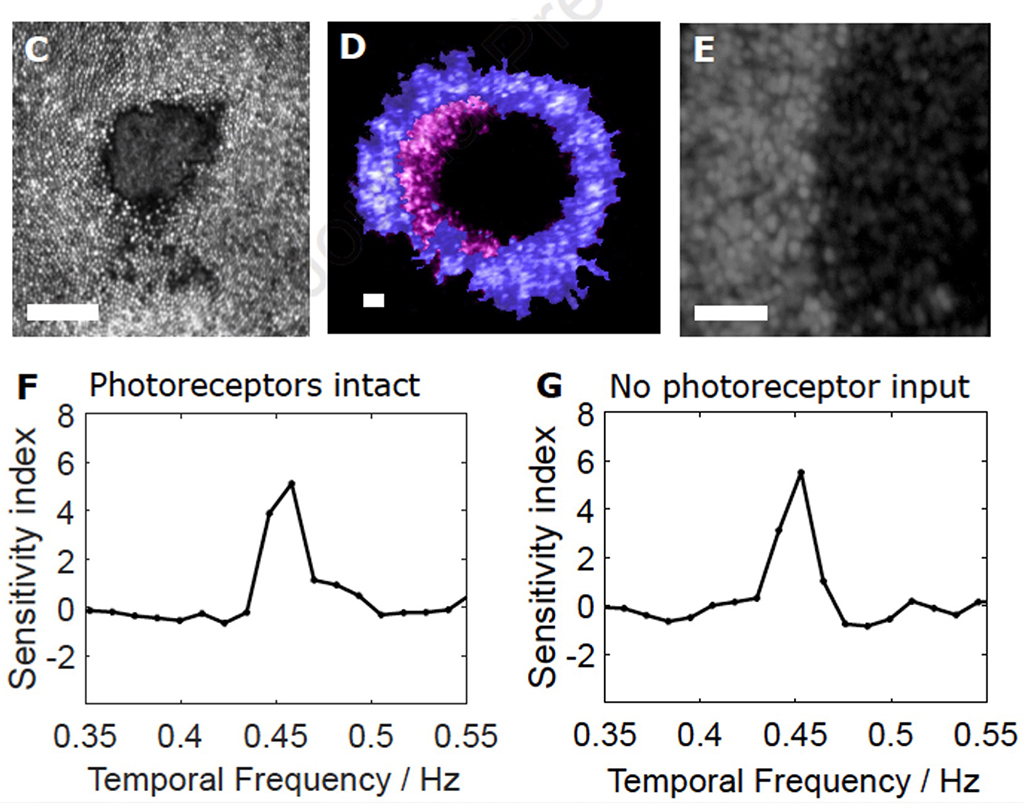
Figure 2: Animal 2-Optogenetic responses 1 year after PRL ablation (From McGregor et al 2021)
This approach has restored robust responsivity in macaque RGCs (McGregor et. al, 2021) at light levels that do not produce phototoxicity, and the restoration has been shown to persist for at least 2 years (McGregor, 2022) as is illustrated in figure 2. 2C shows a reflectance image of lesioned cones, D, the lack of response (purple) of input deprived RGCs, E presence of optogenetic in the RGC ring and F and G, response at 4.5 Hz in normal RGCs and those with no cone input.
Creating a model of vision loss due to outer retina degeneration in macaque
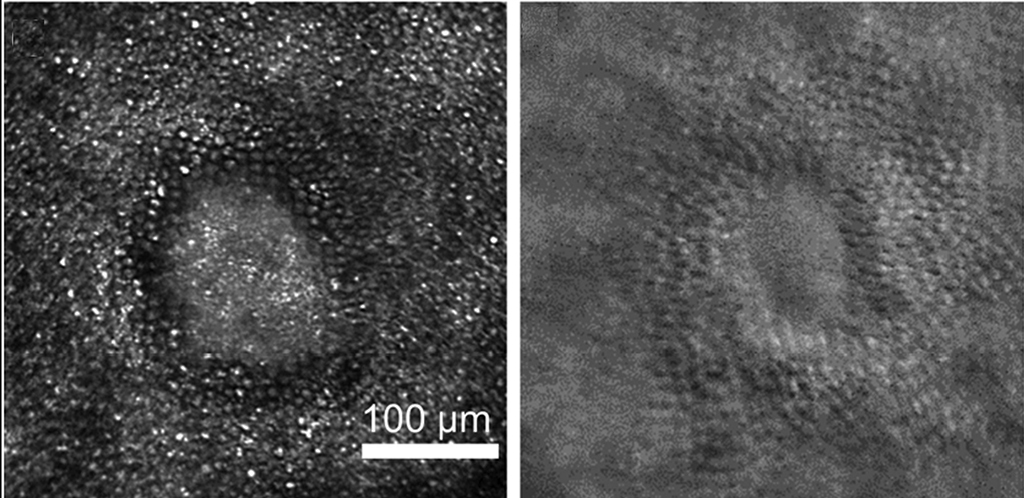
Figure 3: (From Dhakal et al, 2020)
As is well known, there are few naturally occurring outer retina degenerations in the macaque that can provide a substrate to explore vision restoration by optogenetics or transplant of stem cells. The few that have been reported (e.g. McBride 2018) are recently discovered and not sufficiently characterized to be used in research studies, although that may develop in future years. The model we have examined intensively involves selective focal photoreceptor degeneration produced by 2 photon damage to a region of ~ 200 um in the retina. Dhakal et al, 2020 (Figure 3). The left panel of figure 3 shows cone loss in a central region imaged by confocal reflectance imaging, whereas the right portion shows the same region by offset imaging which also shows cone loss in the central region. This model is not identical to actual genetically induced outer retinal damage, but is worth using for several reasons. The first is that this lesion appears to largely involve dense (and typically complete) loss of photoreceptors (largely cones), with little damage to RPE cells or superficial retina (Abouzalideh, 2020).
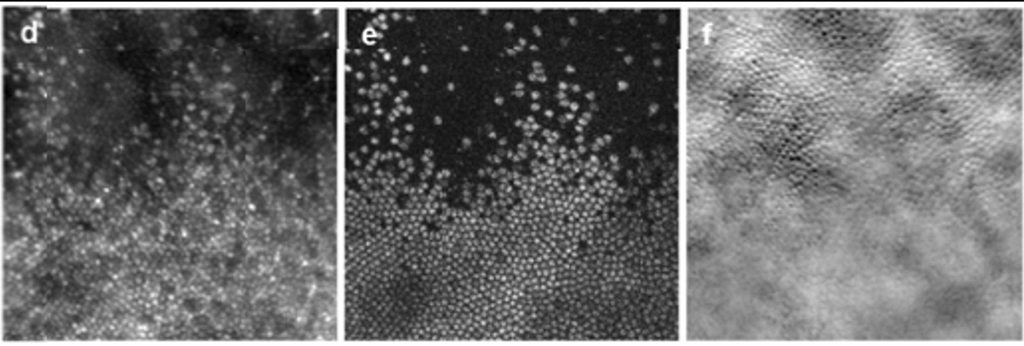
Figure 4: (From Walters et al, 2019)
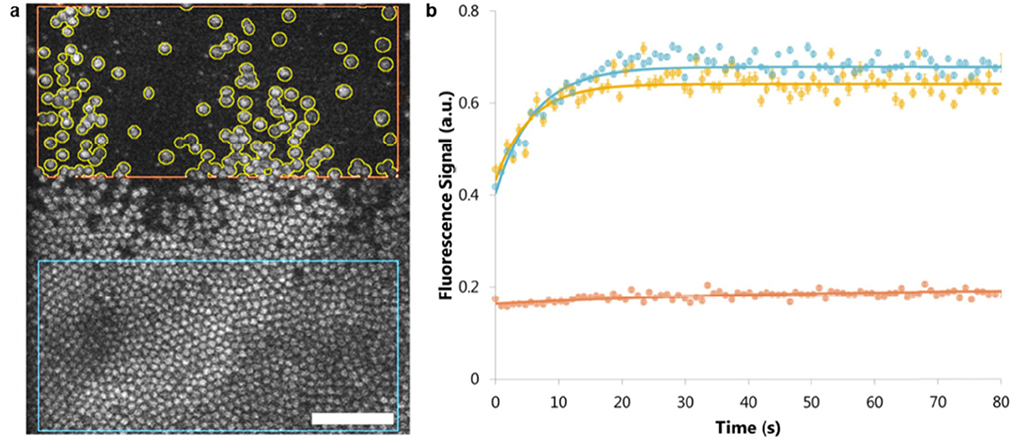
Figure 5: (From Walters et al, 2022)
Figures 4 and 5 demonstrate morphologically (Figure 4 shows a lesioned area in confocal imaging, 2 photon functional imaging and offset imaging) that visual loss is complete at the locus of cone damage.
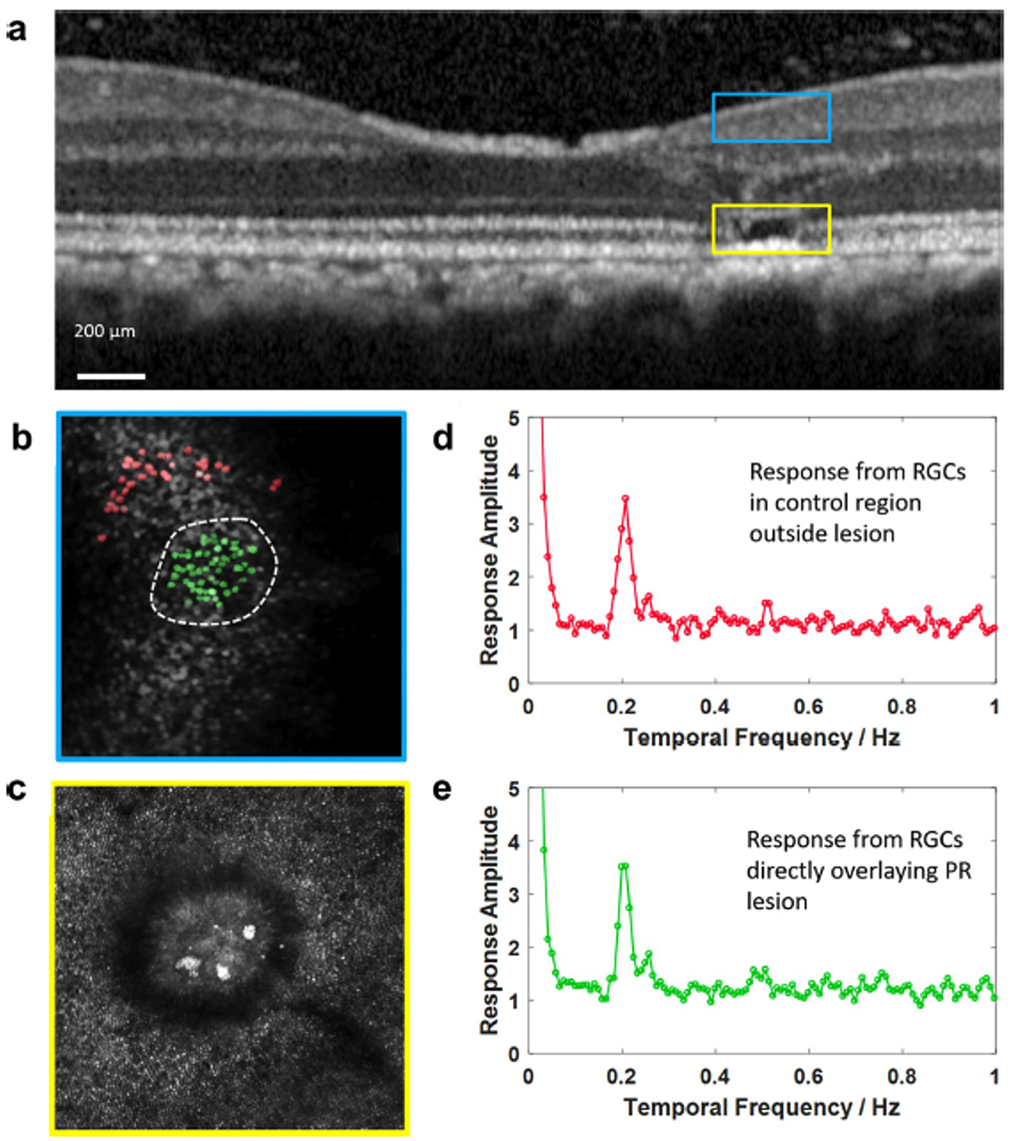
Figure 6: No effect of photoreceptor lesion on responsivity of overlying RCGs (from McGregor et al, 2021)
Although the lesion destroys visual function at the site of cone loss, it does not damage the displaced RGCs that receive input from more central locations (Figure 6). Furthermore, the lesion does not produce a noticeable vision loss to the monkey being studied. The visual function of the monkey appears unaffected to the monkey unless focal visual stimulation is confined to the region of the loss. This somewhat counterintuitive result is confirmed by studies (some from this laboratory) which showed that focal visual loss may not be evident to a subject (in this case a human patient) who sees no visual loss, but is aware that with controlled fixation, function at the tested location may be severely impaired (Merigan Human V4, 1993) while the subject is unaware of any visual loss.
Planned studies with ChRmine
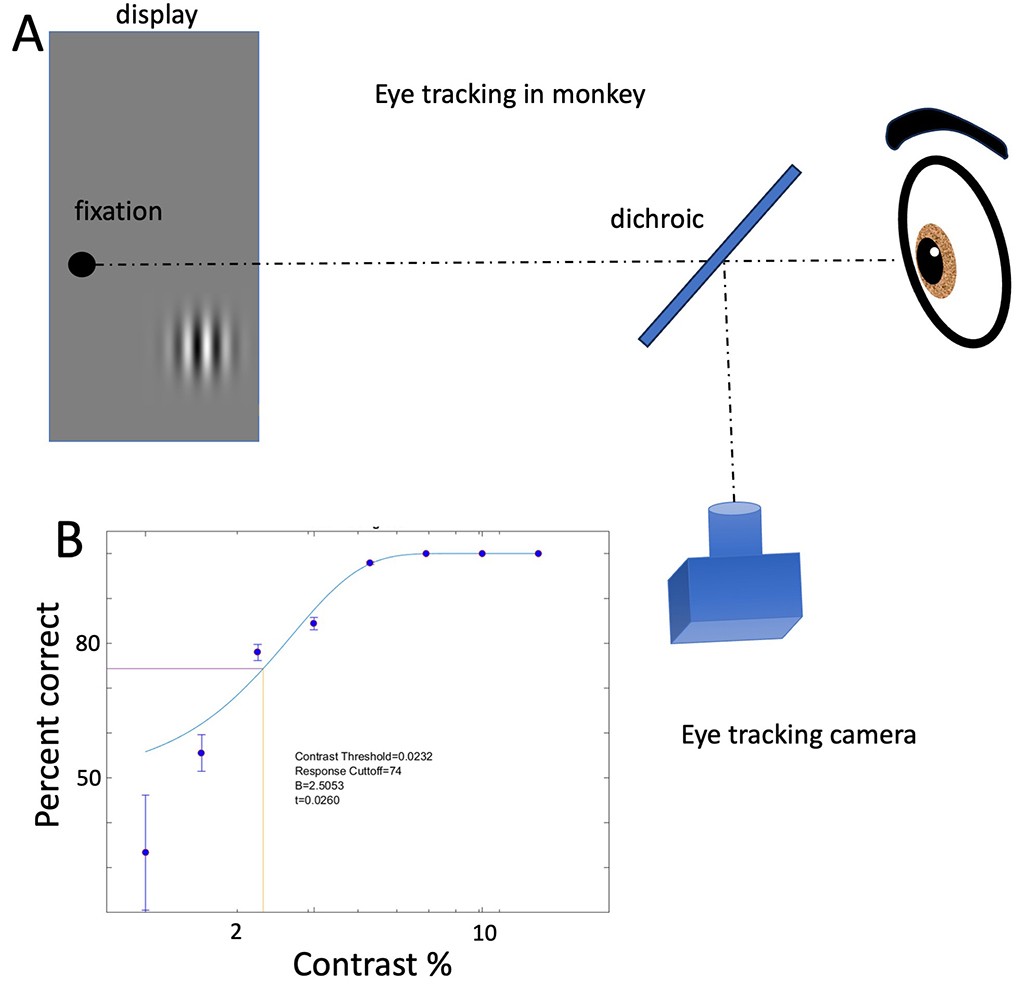
Figure 7
In studies that will be initiated soon, we will measure visual perception in monkeys with vision restored by the novel optogenetic ChRmine (Marchel et al, 2019). Figure 7 shows the method of testing and a typical contrast result.
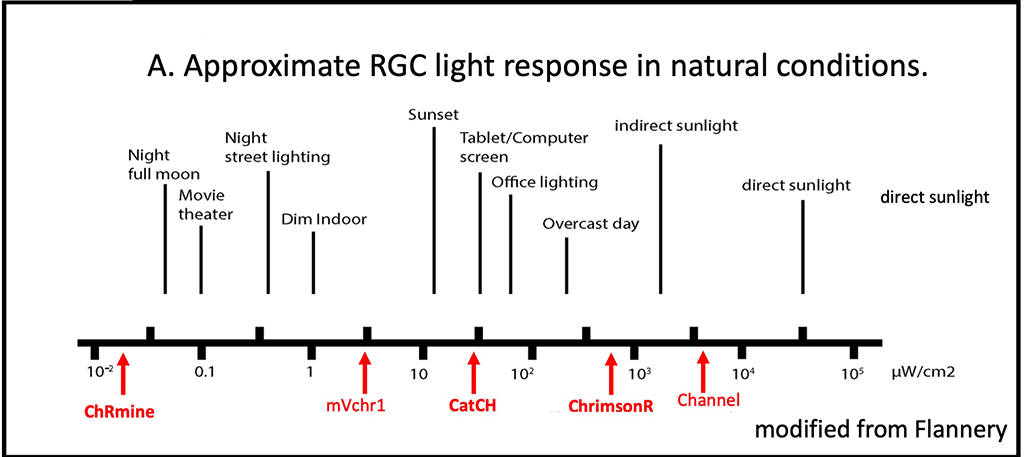
Figure 8: ChRmine
Chrmine is vastly more sensitive than existing optogenetics such as CatCH and ChrimsonR (Figure 8). An important goal of our initial studies is determining the minimal intensity of visual stimuli that gives rise to reliable perception. This result will permit calculation of the optimal expression level (controlled by titer and volume of viral vector injected) for future clinical use in humans low vision patients. As many have noted, it is desirable to minimize expression level to reduce inflammatory changes in the retina, currently a major limitation of retinal gene therapy. As with ongoing studies, examination of ChRmine will examine spatial acuity, contrast sensitivity, motion perception and luminance increment and decrement thresholds.
Funding Sources
Publications
- Walters S, Feeks JA, Huynh KT, and Hunter JJ (2022). Adaptive optics two-photon excited fluorescence lifetime imaging ophthalmoscopy of photoreceptors and retinal pigment epithelium in the living non-human primate eye. Biomed. Opt. Express 13, 389-407. doi:10.1364/BOE.444550. PDF
- McGregor JE, Kunala K, Xu Z, Murphy P, Godat T, Strazzeri JM, Bateman B, Fischer W, Puthussery T, Williams DR, Merigan WH (2021). Optogenetic therapy restores retinal activity in primate for at least a year following photoreceptor ablation. Molecular Therapy, 1525-0016, https://doi.org/10.1016/j.ymthe.2021.09.014. PDF
- McGregor JE, Godat T, Dhakal KR, Parkins K, Strazzeri JM, Bateman BA, Fischer WS, Williams DR, Merigan WH (2020). Optogenetic restoration of retinal ganglion cell activity in the living primate. Nature Communications 11(1), 1703. PDF
- Aboualizadeh E, Phillips MJ, McGregor JE, DiLoreto DA Jr, Strazzeri JM, Dhakal KR, Bateman B, Jager LD, Nilles KL, Stuedemann SA, Ludwig AL, Hunter JJ, Merigan WH, Gamm DM, Williams DR (2020). Imaging Transplanted Photoreceptors in Living Nonhuman Primates with Single-Cell Resolution. Stem Cell Reports. S2213-6711(20)30240-X. doi: 10.1016/j.stemcr.2020.06.019. PDF
- Dhakal KR, Walters S, McGregor JE, Schwarz C, Strazzeri JM, Aboualizadeh E, Bateman B, Huxlin KR, Hunter JJ, Williams DR, Merigan WH (2020). Localized Photoreceptor Ablation Using Femtosecond Pulses Focused With Adaptive Optics. Translational Vision Science & Technology(9), 16. PDF
- Walters S, Schwarz C, Sharma R, Rossi E, Fischer W, DiLoreto D, Strazzeri J, Nelidova D, Roska B, Hunter J, Williams D, and Merigan W (2019). Cellular-scale evaluation of induced photoreceptor degeneration in the living primate eye. Biomed. Opt. Express 10, 66-82. PDF